The ProPep® Nerve Monitoring System is the first FDA-cleared real-time nerve monitoring system for laparoscopic & robotic prostatectomy surgery.
This system helps surgeons identify critical non-visible somatic nerves at risk during surgery, thereby allowing a surgeon to make more-informed decisions on how to spare these nerves, potentially minimizing nerve damage contributing to incontinence and sexual dysfunction.
Clinical Study Shows Clear Benefits
Previous published Patient reported outcomes trials document time to recovery of urinary function can take take 1 to 2 years for a patient to return to baseline after RARP1.
Using the ProPep® Nerve Monitoring System intraoperatively, clinical trial results show that ProPep Patients return to continence much sooner.
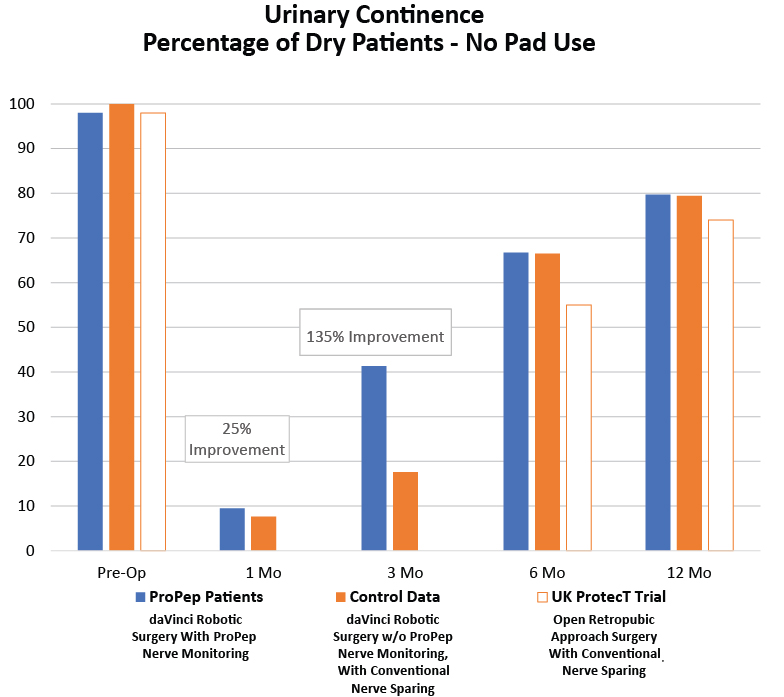
A single, highly-experienced surgeon, prospective cohort study was performed at Indiana University for patients undergoing robotic assisted radical prostatectomy from 2015-2017.
These patients were aligned 1:2 with contemporary controls from the same surgeon on age (within 5 years), biopsy Gleason score, and nerve-sparing status.
ProPep Study Parameters:
Control Group e = 138 men
ProPep Patients = 69 men
The UK ProtecT Trial Parameters: 6-year, 1643-man study, published in the New England Journal of Medicine in September 2016. No data earlier than 6 months post surgery is available.
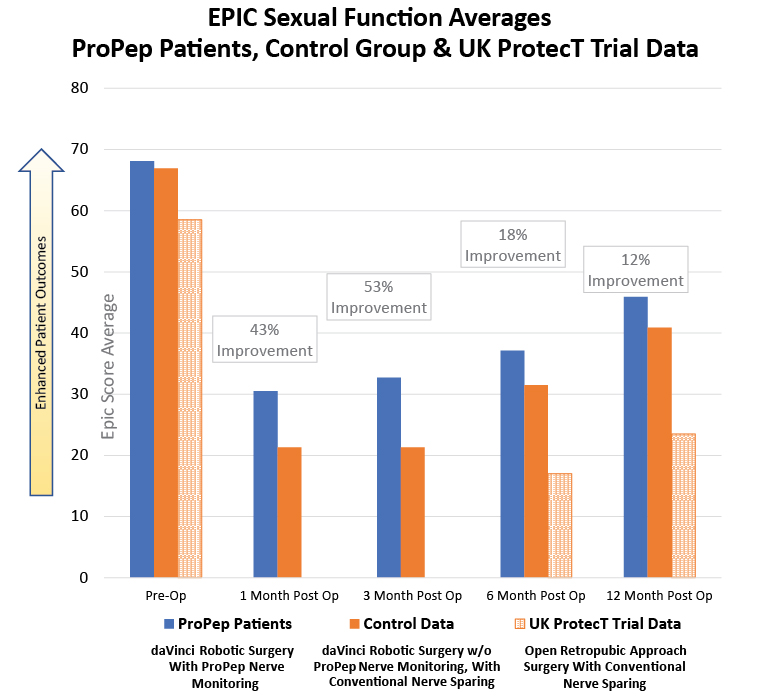
Expanded Prostate Cancer Index composite (EPIC): A one page, patient reported questionnaire consisting of 16 questions that allow the clinician to evaluate a patient. 6 questions relate to the area of Sexual Function.
The EPIC Sexual Function Averages that compare ProPep Patients and the Control data have been adjusted for patient age or co-morbities, factors that might impact sexual function of the men in the this study.
The UK ProtecT Trial Parameters: 6-year, 1643-man study, published in the New England Journal of Medicine in September 2016. No data earlier than 6 months post surgery is available.
*Data on file.
Why Partner?
Monitoring for Urology
FDA-cleared
Over 4000 Cases Completed
IOM Established Reimbursements
Call 512-417-5004 today for product demonstration and training.
Punnen, Sanoj. Long-term Health-related Quality of Life After Primary Treatment for Localized Prostate Cancer: Results from the CaPSURE Registry. European Association of Urology. 2015.
Donovan, J.L. Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. New England Journal of Medicine. 2016.
*ProPep Clinical Study, University of Indiana, Department of Urology, 2018. Data on file.
 Patients and the ProPep Advantage
Patients and the ProPep Advantage